Paracetamol Fail Quality Test: 53 medicines including paracetamol have failed the quality test. These include vitamins, sugar and blood pressure medicines as well as antibiotics. The country’s largest drug regulatory body Central Drugs Standard Control Organization (CDSCO) has released its list.
The CDSCO list includes calcium and vitamin D3 supplements, anti-diabetes pills and high blood pressure medicines.
Table of Contents
The list of banned drugs also includes Clonazepam tablets used for seizures and anxiety, painkiller Diclofenac, Ambroxol used for respiratory illness, anti-fungal Fluconazole and some multivitamins and calcium tablets.
These medicines are manufactured by big companies like Hetero Drugs, Alkem Laboratories, Hindustan Antibiotics Limited (HAL), Karnataka Antibiotics and Pharmaceuticals Limited.
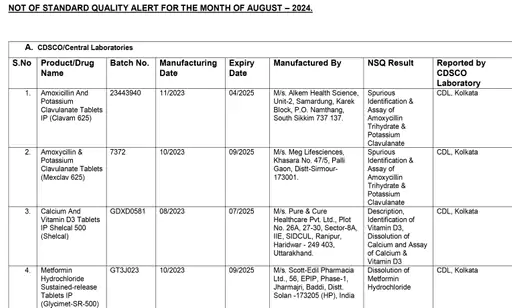
CDSCO released a list of 48 medicines
Metronidazole, a medicine given for stomach infection and manufactured by Hindustan Antibiotics Limited, also failed this test. Similarly, Shelcal tablets of Torrent Pharmaceuticals also failed the test.
CDSCO had done quality tests of 53 medicines, but released the list of only 48 medicines. Because out of 53, 5 medicine manufacturing companies said that these are not their medicines, but fake medicines are being sold in the market in their name. After this, they were removed from the list.
Click here to see the list of all 48 medicines…
In August, the central government banned 156 fixed dose combination drugs
In August this year, the central government banned 156 fixed dose combination (FDC) drugs. These were commonly used for fever and cold as well as painkillers, multi-vitamins and antibiotics.
The government said that there is a possibility of danger to humans due to their use. Therefore, the production, consumption and distribution of these medicines will be banned across the country.
The government had issued this order on the recommendations of the Drugs Technical Advisory Board. The board said in its report that there is no medical justification for the ingredients present in these FDC medicines.
Medicines made by mixing more than one drug in a single pill are called fixed dose combination drugs (FDC), these drugs are also known as cocktail drugs.
Hair treatment, skincare and anti-allergic medicines also included
According to the order issued by the central government, the use of amylase, protease, glucoamylase, pectinase, alpha galactosidase, lactase, beta-gluconase, cellulase, lipase, bromelain, xylanase, hemicellulase, malt diastase, invertase and papain is likely to pose a risk to humans.
The list of banned medicines includes medicines used for hair treatment, antiparasitic (used in parasitic infections), skincare, anti-allergic. The government said that there are other medicines available in the market in place of these medicines. There will be no ban on them.
Samples of 10 medicines of Chief Minister’s Free Medicine Scheme fail in Rajasthan
In May this year, samples of 10 medicines supplied under the Chief Minister’s Free Medicine Scheme in Rajasthan failed. Rajasthan Medical Services Corporation (RMSC) has banned the supply of 10 medicines of 8 companies. These medicines include tablets used for fungal infection, injections given to serious malaria patients, eye drops and Asthalin, a medicine used in case of breathing problems.
Also Read:

